The dose rate is calculated from the concentrations of radioactive elements in the sample and its surroundings (only the U and Th decay chains and the 40K-decay are of relevance; a minor contribution comes from 87Rb in the sediment) plus a component of cosmic rays. There are three different ionizing rays, which are emitted from radioactive elements (Figure 4):
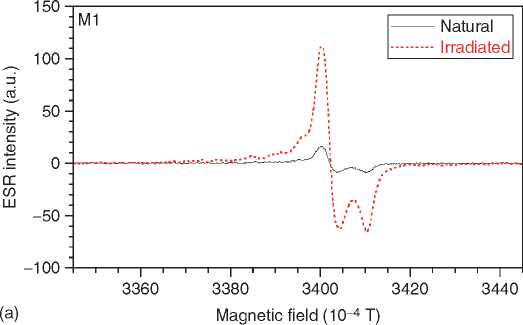
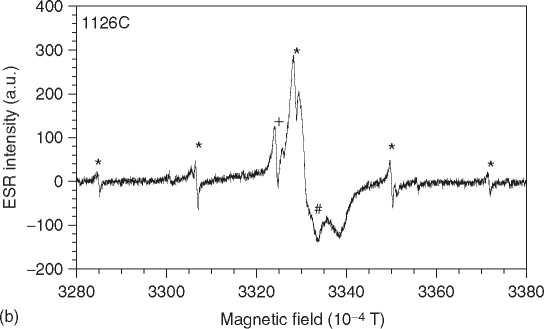
Figure 3 ESR spectra of tooth enamel. The spectrum is dominated by a CO2 radical (a). Some spectra show interferences by methyl (* in b), SO2 (+), and a rotating CO2 (#).
• gamma rays (photons) have a range of about 30 cm;
• beta rays (electrons) have a range of about 2 mm; and
• alpha rays (helium nuclei) have only a very short range of about 20-40 pm because of the large size of the particles.
Alpha particles are less efficient in producing ESR intensity than beta and gamma rays. Therefore, an alpha efficiency, which is usually in the range of 0.13 ± 0.02, has to be determined.
The concentrations of radioactive elements within a sample are usually very different from their surroundings. Thus, ‘internal dose rates’ and ‘external dose rates’ have to be assessed independently. Furthermore, it is necessary to estimate the cosmic dose rate, which is about 300 pGyyr21 at sea level and decreases with depth below ground, and is also dependent on altitude as well as on geographic latitude. The conversion of the elemental analysis into dose rates is shown in Table 1. Dose rate calculations become more complicated when disequilibrium in the U-decay chains or attenuation factors has to be considered.

 World History
World History









