Lipid residues are the primary constituents of most absorbed ceramic residues. They are comprised of
Various classes of lipid, including acyl lipids, terpenoids, waxes, alkanes, and alkanols. Lipid residues must first be extracted from their matrix, whether it is an absorbed or visible residue. They must then be analyzed and interpreted. The three primary types of interpretation involve biomarkers, relative abundances, and compound-specific stable isotope analysis. Relative abundances of components can also be useful in interpreting residues, but must be used carefully, due to the effects of environmental degradation. Even with this caution, however, residues comprised primarily of plant-based components contain more unsaturated fatty acids relative to unsaturated fatty acids, as shown in Figure 2, while those originating from animal-based materials contain more unsaturated fatty acids, and occasionally the presence of the biomarker cholesterol.
Extraction
The standard method for extraction of absorbed ceramic residues was set forth by Evershed et al. in 1992. It involves powdering a potsherd in a mortar and pestle, and extracting the organic constituents in a strong solvent. The solvent is then removed from the powdered matrix and blown down to produce a pure residue. Although experiments have been undertaken on nondestructive analyses of absorbed residues, the destructive approach has so far been more effective. The extraction of visible residues is based on the standard methodology for absorbed residues, with the use of smaller amounts of solvent.
Types of Analysis
Various types of instrumental analyses have been applied to residue analysis. For residues with a complex mixture of components, which includes most absorbed residues, some sort of chromatographic separation of constituents prior to identification is preferable. The standard approach involves the use of a GC/MS. Liquid chromatography (LC/MS) produces a similar effect, and works well on less volatile constituents of a residue, though less well on more volatile constituents. Both types of analyses produce a chromatogram, shown in Figure 2, which indicates the various separated components and their relative abundance in the form of peaks. Each peak in the chromatogram has a corresponding mass spectrum, such as that in Figure 3, which allows the identification of component. The extracted residue is usually split into different fractions, which are processed and derivatized in various ways to maximize the desired information. Usually, a total lipid extract is analyzed by GC/MS in order to view the entire residue and its state of preservation.
Visible residues are amenable to a wider range of analyses than absorbed residues, as the carbonized residue may undergo Fourier-Transform Infrared Spectrometry (FT-IR) which allows the detection of components that cannot successfully undergo chromatographic analysis. Microscopic analysis of visible residues is also useful, sometimes permitting the identification of starch grains and macroscopic remains embedded in the residue.
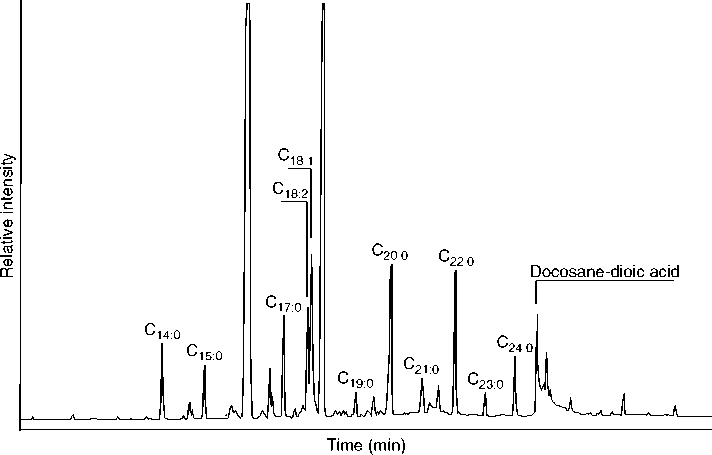
Figure 2 A gas chromatogram of the fatty acid methyl ester fraction of an absorbed residue extracted from a potsherd. The subscripts refer to number of carbons followed by the number of double bonds (unsaturation) present; C18:2 refers to an eighteen-carbon chain fatty acid with two double bonds. The relatively high abundance of unsaturated fatty acids in this residue suggest a primarily plant origin for the residue.
Compound-specific Isotope Analysis
Compound-specific isotope analysis is very useful in areas where the biome includes both C3 and C4 photosynthesis, either naturally or through importation of crops. A good example of this is the identification of maize in absorbed pottery residues. The diet in southeastern and Midwestern North America is primarily C3, with very few CAM or C4 plants present in the diet except for the imported tropical grass maize. Maize does not have a biomarker, but it is abundant in the alkanol «-dotriacontanol. Compound-specific isotope analysis of a residue can determine if the «-dotriacontanol is C3 or C4 in origin, as shown in Figure 4. If the component is C4
In origin, it is reasonable to argue that maize was processed in the pot.

 World History
World History









